RESUMEN
Los agentes utilizados en blanqueamiento dental provocan una respuesta inflamatoria de la pulpa, que depende de la concentración y el tiempo de aplicación de la sustancia empleada. Objetivo: Evaluar el nivel de penetración del blanqueamiento a base de peróxido de carbamida a diferentes concentraciones: 10, 20 y 35% dentro de la cámara pulpar. Materiales y métodos: Se utilizaron 120 terceros molares humanos extraídos, divididos aleatoriamente en cuatro grupos (n=30): grupo A: control; grupo B: 10% gel de Peróxido de Carbamida (CP del inglés Carbamide Peroxide); grupo C: 20% gel CP y grupo D: 35% gel CP. Los dientes se cortaron 2 mm por debajo del límite amelocementario con una máquina de corte, se colocó buffer de acetato en la cámara pulpar y se aplicó por 40 minutos el agente clareador una sola vez. Los dientes se mantuvieron a temperatura ambiente (25°C) durante el proceso. La penetración de CP se estimó con cristal violeta y peroxidasa de rábano picante, en un espectrofotómetro de absorbancia. Los datos fueron analizados mediante las pruebas estadísticas ANOVA complementada con el Test post Hoc de Tukey con un grado de significancia al 5%. Resultados: Las medias obtenidas para el Grupo B fueron de 0,062 mg (±0,018), para el Grupo C fueron de 0,063 mg (±0,017), y para el Grupo D fueron de 0,086 mg (±0,024). Existiendo diferencia significativa (p= <0.05) del grupo D con relación a los otros grupos. Conclusión: la penetración de CP en la cavidad pulpar depende de la concentración, siendo mayor en concentración al 35%.
Palabras clave: Cavidad Pulpar, Pulpa Dental, Blanqueamiento de dientes, Peróxido de Carbamida; Peróxido de Hidrógeno.
ABSTRACT
The elimination of calcium hydroxide in the root canal is decisive for the success of endodontic treatment, the remnants can interact negatively with endodontic sealants increasing filtrations and decreasing the quality of the seal. Objective: To evaluate the effect of intra-duct medication with calcium hydroxide paste on the penetration of the sealing cement inside the dentinal tubules. Materials and methods: 20 distal roots of upper molars were instrumented using the Wave One Large 40 / .08 System. They were randomly divided into two groups: one sealed with a single cone technique and Ah plus cement with rhodamine-B and another sealed with the same technique and Ah plus cement with rhodamine B, previous placement for 15 days and removal by recapping the paste calcium hydroxide. Subsequently, the teeth were cut transversely and photomicrographs of the cervical, middle and apical third were performed using the laser scanning confocal microscopy technique. The maximum depth of penetration was determined through the Image J program. Results: The Ah plus sealing cement had lower penetration values when the calcium hydroxide paste was previously used as an intra-channel medication (p <0.01). The third of the duct with the highest penetration was the cervical third followed by the middle third and finally the apical (p <0.01). Conclusion: Remaining calcium hydroxide decreases the penetration of the sealing cement Ah plus in the dentinal tubules in all thirds of the root canal.
Keywords: Calcium Hydroxide; Sealing cement; Tubular penetration; Confocal laser microscopy.
RESUMO
Os agentes utilizados no clareamento dos dentes estimulam uma resposta inflamatória da polpa, o que depende da concentração e do tempo de aplicação da substância utilizada. Objetivo: Avaliar o nível de penetração do clareamento à base de peróxido de carbamida em diferentes concentrações: 10, 20 e 35% no interior da câmara pulpar. Materiais e métodos: foram utilizados 120 terceiros molares humanos extraídos, divididos aleatoriamente em quatro grupos (n = 30): grupo A: controle; grupo B: gel de peróxido de carbamida a 10% (CP do inglês carbamide peroxide); grupo C: gel CP de 20% e grupo D: gel CP de 35%. Os dentes foram cortados 2 mm abaixo do limite amelocementário com uma máquina de corte, tampão acetato foi colocado na câmara pulpar e o clareador foi aplicado por 40 minutos apenas uma vez. Os dentes foram mantidos à temperatura ambiente (25 °C) durante o processo. A penetração de CP foi estimada com cristal violeta e peroxidase de rábano picante, em espectrofotômetro de absorvância. Os dados foram analisados por meio dos testes estatísticos ANOVA, complementados com o teste post hoc de Tukey, com um grau de significância de 5%. Resultados: As médias obtidas no grupo B foram de 0,062 mg (± 0,018), no grupo C foram de 0,063 mg (± 0,017) e no grupo D foram de 0,086 mg (± 0,024). Existe uma diferença significativa (p = <0,05) do grupo D em relação aos demais grupos. Conclusão: a penetração da PC na cavidade pulpar depende da concentração, sendo maior na concentração em 35%.
Palavras-chave: Cavidade Pulpar, Polpa Dentária, Clareamento Dentário, Peróxido de Carbamida; Peróxido de hidrogênio.
INTRODUCTION
Tooth whitening is a chemical procedure that acts on the dental structure in order to improve the microsesthetics, however, the interaction of the bleaching agent causes harmful effects on the dental pulp such as sensitivity, reversible pulpitis and cell death due to necrosis1–4.
Carbamide peroxide is a bleaching agent that, upon contact with saliva, breaks down 33% of its hydrogen peroxide content, an oxidizing agent that gives rise to reactive oxygen species (ROS) with the ability to degrade molecules Organic complexes present in the dental structure5,6. In addition, carbamide peroxide releases urea, which breaks down into carbon dioxide and ammonia, substances that have proteolytic properties that increase the effectiveness of tooth whitening6–9.
However, oxidative reactions and cell damage caused by free radicals are responsible for the toxicity of bleaching agents10–12. The low molecular weight of Hydrogen Peroxide has the ability to diffuse through the enamel and dentin to reach the pulp space, leading to harmful effects from the inflammatory reaction of the pulp to large areas of tissue necrosis, depending on the concentration of the agent bleach, application time, heat, or if it is a young tooth5,10,13.
For this reason, this research allowed us to assess the level of Carbamide Peroxide in its different concentrations on the pulp chamber, the results obtained serve as scientific information and clinical criteria to be considered by professionals when performing said procedure.
Materials and methods
This study was approved by the Ethics Subcommittee of the Faculty Research Commission of the Central University of Ecuador with the code 0160-FO-G-2019.
For this study, 120 extracted human third molars were used, randomly divided into four groups (n = 30 / group): group A: control; group B: 10% CP gel; group C: 20% CP gel and group D: 35% CP gel.
Sample cleaning phase
The samples were subjected to a cleaning and debris removal process using a brand ultrasonic tip (Woodpecker, London, England) for later storage in 0.9% saline solution, at room temperature to avoid dehydration. (See Figure 1)

Figure 1. Cleaning and removing debris (Ultrasonic tip)
For the experiment, the dental pieces were cut 2 mm below the amelocementary limit, this process was carried out with an Abrasive cut-off Wheels cutting machine (BUEHLER LTD, Essligen, Germany), with constant irrigation to avoid overheating the tooth. (See Figure 2)

Figure 2. Cutting the dental part with constant irrigation
Preparación de la muestra
La cavidad pulpar fue ampliada y preparada con la fresa endozeta (Maillefer, Suiza) hacia la pared lingual con el fin de establecer una estructura dental intacta de 2 mm de grosor que podría contener 40 mL de tampón de acetato, compuesto por ácido acético y acetato de sodio. Posteriormente, las fosas y fisuras oclusales fueron selladas con resina fluida (Filtek TM Z350XT, 3M ESPE, Saint Paul, USA) para evitar cualquier fuga del tampón fuera de la cavidad. Además, se colocó una etiqueta adhesiva circular de 5 mm de diámetro en el centro de la superficie labial. Luego, la porción restante del diente se pintó con esmalte de uñas gris (Nailwear pro, AVON, Quito, Ecuador) y después del secado se retiró la etiqueta adhesiva (eticoll, Girona, España), dejando un área de esmalte sin pintar de tamaño estándar para la aplicación del Blanqueamiento13. Después, se fabricó una plantilla de silicona de adición (elite HD+, Zhermack, Badia Polesine, Italia) colocando la superficie lingual en un ángulo de 30° desde la base. (Ver Figura 3)

Figure 3. Prepared sample
Whitening
On the vestibular face of each tooth, was placed a continuous layer (one drop) of Carbamide Peroxide (Opalescence tooth whitening systems, Ultradent Products Inc., South Jordan, USA) at 10%, 20% and 35% on 30 teeth, for a time of 40 minutes only once. In addition, it was covered with a linear low density polyethylene wrap (packaging, Valencia, Spain) to simulate the placement of a semi-rigid acetate splint. Subsequently, 40 µL of acetate buffer (pH: 4.5) was placed in the pulp cavity, this solution has the ability to prevent peroxide degradation until the moment of analysis. At the end of this time the teeth were rinsed with plenty of water13. The teeth were kept at room temperature (25°C) during Whitening. (See Fig. 4)
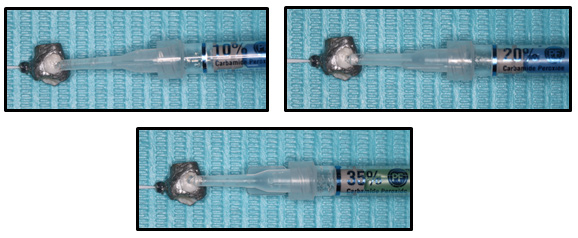
Figure 4. Placement of Carbamide Peroxide Opalescence tooth Whitening systems (Ultradent Products, Inc.) at a) 10%, b) 20% and c) 35% gel, 40 minutes each
CP penetration measurement to the pulp chamber
A stock solution was prepared for the 120 samples, containing: 1mL of acetate buffer (pH: 4.5), 12.5 mL of violet crystal solution (0.0313 mg / ml), 25 mL of phosphate buffer (pH: 6.17), and 12.5 mL of horseradish peroxidase solution (0.0625 mg / ml)13.
For the measurement of peroxide in the pulp chamber, 10 µL recovered from the pulp cavity was transferred to a cell containing 2.5 ml of phosphate buffer and 0.5 ml of the stock solution (final solution)13. (See Figure 5)

Figure 5. Placing 10 µl of acetate buffer from the cavity to the final solution
To carry out the process mentioned above, each study group was divided into 10 subgroups consisting of three teeth to avoid excessive dilution of the stock solution, for each sample the measurement was performed four times by means of the spectrophotometer, as follows: the initial absorbance of the final solution without buffer in the cavity was measured 4 times, then 10 µL of the tooth cavity 1 was placed and the absorbance of said tooth was measured 4 times, then 10 µL of the tooth cavity 2 was placed and the absorbance of said tooth was measured 4 times, and finally 10 µL of the tooth cavity 3 was placed and the absorbance of said tooth was measured 4 times, the average of each one was subsequently calculated, and with these values the delta absorbance values (ΔA) subtracted the dilution factor13. Then the same process was carried out in another cell with the remaining subgroups until the 30 samples of each group were completed. (See Figure 6)

Figure 6. Placing the cell in the specimen compartment of the Spectrophotometer
The color intensity was measured by an absorbance spectrophotometer (CARY 50 BIO UV-VISIBLE, VARIAN, Saumur, France) at a wavelength of 591 nm. A standard calibration curve with known amounts of peroxide was used to determine the amount in equivalent milligrams in the samples. Using the calibration curve, a quadratic equation of the second degree was obtained, and since it had a coefficient of determination (R2) of 0.9843, the apparent concentrations in the pulp chamber were determined. Where the equation would be: 1.9089x2 -1.9898x + ΔA + 0.023 = 0 (See Chart 1).
The delta absorbance values (ΔA) were replaced in the equation, the result was multiplied by four, since 40 uL was placed in the pulp chamber in total. In this way the amount of Carbamide peroxide that entered the pulp chamber was calculated.

Graphic 1. Calibration curve
It is important to emphasize that the oxidation of the dye did not affect absorbance, for this purpose several dilutions were made, using gentian violet and peroxide, where it can be determined that the variations are minimal, and practically constant from one dilution to another.
Spectral scanning: It is worth mentioning that, in order to know the maximum wavelength of the present study, a scan was made in the visible spectrum (400 nm to 800nm) with the help of the UV-Visible CARY 50 BIO Spectrophotometer (VARIAN, France) to the solution final.
Results
From the experimental data, the information was organized in an Excel table, with these values a database was built in SPSS version 24 in Spanish IBM ®, then the Kolmogorov-Smirnov Normality test was carried out where the values of Significance (Sig) is greater than 0.05 (95% reliability), therefore, the samples come from populations with normal distribution, directing the analysis for the parametric tests: ANOVA, complemented with the Tukey post Hoc test with a significance 95%.
According to the descriptive statistics, the means obtained are: for CP at 10%: 0.062 mg (± 0.018), for 20%: 0.063 mg (± 0.017), and for 35%: 0.086 mg (± 0.024). (See graph 2)

Graphic 2. Comparison of means (mg)
From the ANOVA test, supplemented with the Tukey post Hoc test, it was determined that the control group did not detect Carbamide Peroxide in the pulp chamber, so it was not taken into account. However, the values of the group in which 10% PC was used did not differ significantly from the group that used the 20% PC, but these groups did differ from the group in which the 35% concentration was used. (See table 1)

Table 1. Pruebas post hoc (Prueba de Tukey)
Discussion
Currently there is a great demand for teeth whitening that improve the physical appearance of people, however, there are harmful effects on dental structures, especially on dental pulp, which is related to the concentration of the bleaching agent and the application time5,6,10,14, so the objective of this study was to evaluate the penetration into the pulp chamber of the CP at different concentrations: 10%, 20% and 35%, in order to assess the peroxide level that went through the pulp tissue. It was shown that the penetration of the CP into the pulp chamber depends on its concentration.
As demonstrated in the present investigation, the penetration of the CP into the pulp chamber can generate pulp inflammation. From Oliveira et al. (2014), mention about the intense oxidative stress caused by bleaching gels, and against this the inability of cellular protection mechanisms such as the production of antioxidant enzymes to eliminate local ROS; and cell membrane damage caused by lipid peroxidation, which results in the destruction of cell function and cell death due to necrosis. Also, proteolytic enzymes present in dentin are activated, such as cathepsins and metalloproteinases, causing degradation of the pulp's extracellular matrix. Therefore, depending on the intensity of cellular damage and alterations of the extracellular matrix, the effects range from the inflammatory reaction of the pulp to large areas of tissue necrosis5.
De Oliveira and cols. (2014), showed that the low toxicity of 10% Carbamide Peroxide is related to the lower diffusion of peroxide through dental structures, which is why it is considered the safest concentration for pulp tissue5, coinciding with the present study where 10% Carbamide Peroxide showed less penetration into the pulp chamber.
Patri and cols. (2013), evaluated the penetration of 10% Carbamide Peroxide in restored teeth, concluded that there is penetration of the bleaching agent into the pulp chamber15, although the method presented used intact teeth, the same conclusion was reached.
The penetration of the bleaching agent seems independent of the substance used, Patri and cols. (2016) and Costa and cols. (2014) used 30% and 38% Hydrogen Peroxide as a bleaching agent, respectively, they concluded that there is diffusion of peroxide to the pulp chamber10,16. Although we use 10% Carbamide Peroxide as a bleaching agent, 20% and 35% obtained the same conclusion; probably because the CP decomposes into hydrogen peroxide, acting in a similar way, so that the penetration of peroxide into the pulp chamber at the three concentrations evaluated is possible.
De Oliveira and cols. (2014) in their research they analyzed the relationship between the concentration of the bleaching agent and the diffusion of peroxide, reaching the conclusion that there is greater diffusion of 35% Hydrogen Peroxide than 10% Carbamide Peroxide5. In our case, 10%, 20% and 35% Carbamide Peroxide was used as a bleaching agent, obtaining the same conclusion, that the greater the concentration, the greater the penetration into the pulp chamber.
On the other hand, Marson and cols. (2015) determined that the penetration of Hydrogen Peroxide depends on the contact time of the whitening gel with the dental structure regardless of the concentration of the peroxide, resulting in that in concentrations of 35% Hydrogen Peroxide there was greater penetration into the pulp chamber than in concentrations of 38% Hydrogen Peroxide in 45 minutes17. The present study states that the amount of peroxide diffusion into the pulp chamber depends on its concentration by applying the bleaching agent 40 minutes; the variation in the data obtained may be due to the fact that bleaching gels maintain more than 86% of their initial peroxide concentration after 45 minutes, thus maintaining their bleaching capacity17.
Regarding the method for quantifying peroxide in the pulp cavity, Gomes and cols.. (2010) in their study used the one proposed by Bauminger18 and modified by Hannig and cols.19 which is a spectrophotometric analysis based on the reaction of 4-aminoantipyrine and phenol with peroxidase-catalyzed Hydrogen Peroxide20. In addition, Soares and cols.. (2015) and Patri and cols.. (2016) in their investigations they use the Mottola method that is based on the reaction of Hydrogen Peroxide with violet leucocristal catalyzed by the horseradish peroxidase enzyme16,21. However, in the present study it was carried out with the violet crystal dye and the horseradish peroxidase enzyme. Despite using a different methodology, it was observed that there was a positive reaction of peroxide with peroxidase, concluding in our study that the greater the concentration of the bleaching agent, the greater the penetration into the pulp tissue.
Conclusions
According to the results obtained in the study, we conclude that in tooth whitening treatments with Carbamide Peroxide there is penetration of peroxide into the pulp chamber, being greater in Carbamide Peroxide at 35%, so the hypothesis is accepted research. When comparing the penetration amount of 10%, 20% and 35% Carbamide Peroxide into the pulp chamber, it is concluded that the higher the concentration of the peroxide, the greater the diffusion into the pulp chamber, with 35% Carbamide Peroxide being statistically significant.
Bibliografía
- Cedillo Orellana SI. Efectos del blanqueamiento dental sobre el tejido pulpar. 2016;1–46. Available from: http://dspace.ucuenca.edu.ec/handle/123456789/24808
- Cedeño J. Creencias y mitos del blanqueamiento dental en los habitantes de la parroquia San Mateo periodo 2016-2017. 2017;9:1–50.
- Batista G, Barcellos D, Torres C, Goto E, Pucci C, Borges A. The Influence of Chemical Activation on Tooth Bleaching Using 10% Carbamide Peroxide. Oper Dent [Internet]. 2011;36(2):162–8. Available from: http://www.jopdentonline.org/doi/10.2341/09-280-L
- Demarco FF, Meireles SS, Sarmento HR, Dantas RVF, Botero T, Tarquinio SBC. Erosion and abrasion on dental structures undergoing at-home bleaching. Clin Cosmet Investig Dent. 2011;3:45–52.
- de Oliveira Duque CC, Soares DG, Basso FG, Hebling J, de Souza Costa CA. Bleaching effectiveness, hydrogen peroxide diffusion, and cytotoxicity of a chemically activated bleaching gel. Clin Oral Investig. 2013;18(6):1631–7.
- Goldberg M, Grootveld M, Lynch E. Undesirable and adverse effects of tooth-whitening products: A review. Clin Oral Investig. 2010;14(1):1–10.
- Hyland BW, McDonald A, Lewis N, Tredwin C, Petrie A, Hall S, et al. A new three-component formulation for the efficient whitening of teeth (Carbamide Plus). Clin Oral Investig. 2014;19(6):1395–404.
- Borges B, Borges J, de Melo C, Pinheiro I, Santos A dos, Braz R, et al. Efficacy of a Novel At-home Bleaching Technique With Carbamide Peroxides Modified by CPP-ACP and Its Effect on the Microhardness of Bleached Enamel. Oper Dent [Internet]. 2011;36(5):521–8. Available from: http://www.jopdentonline.org/doi/10.2341/11-013-L
- Hirata R, Higashi C. Blanqueamiento dental: Concepto y sustancias blanqueadoras. Médica Pan. 2012. 385 p.
- Costa CAS, Riehl H, Kina JF, Sacono NT, Hebling J. Human pulp responses to in-office tooth bleaching. J Esthet Restor Dent [Internet]. 2014;26(5):356. Available from: http://dx.doi.org/10.1016/j.tripleo.2009.12.002
- Baxter R, Hastings N, Law A, Glass EJ. Blanqueamiento Dental. Anim Genet. 2008;39(5):561–3.
- Solís Cessa E. Aclaramiento dental: revisión de la literatura y presentación de un caso clínico. Dental clearance: review of the literature and case report. Rev ADM [Internet]. 2018;75(1):9–25. Available from: www.medigraphic.com/adm
- Kwon S, Pallavi F, Shi Y, Oyoyo U, Mohraz A, Li Y. Effect of Bleaching Gel Viscosity on Tooth Whitening Efficacy and Pulp Chamber Penetration: An In Vitro Study. Oper Dent [Internet]. 2018;43(3):326–34. Available from: http://www.jopdentonline.org/doi/10.2341/17-099-L
- Reis A, Kossatz S, Martins G, Loguercio A. Efficacy of and Effect on Tooth Sensitivity of In-office Bleaching Gel Concentrations: A Randomized Clinical Trial. Oper Dent [Internet]. 2013;38(4):386–93. Available from: http://www.jopdentonline.org/doi/10.2341/12-140-C
- Patri G, Agnihotri Y, Rao SR, Lakshmi N, Das S. An in Vitro Spectrophotometric Analysis of the Penetration of Bleaching Agent into the Pulp Chamber of Intact and Restored Teeth. 2013;7(12):3057–9.
- Patri G, Acharya G, Agrawal P, Panda V. Spectrophotometric evaluation of the pulpal peroxide levels in intact and restored teeth - An invitro study. J Clin Diagnostic Res. 2016;10(8):ZC44–7.
- Marson F, Goncalves R, Silva C, Cintra L, Pascotto R, Santos P, et al. Penetration of Hydrogen Peroxide and Degradation Rate of Different Bleaching Products. Oper Dent. 2015;72–9.
- Bauminger BB. Micro method for manual analysis of true glucose in plasma without deproteinization. 1974;1015–7.
- Hannig C, Za R, Henze E, Dreier S, Attin T. Peroxide release into saliva from five different home bleaching systems in vivo. 2005;18(1):15810475.
- Gomes CR, Wiegand A, Sener B, Attin T. Influence of chemical activation of a 35% hydrogen peroxide bleaching gel on its penetration and efficacy - In vitro study. J Dent [Internet]. 2010;38(10):838–46. Available from: http://dx.doi.org/10.1016/j.jdent.2010.07.002
- Soares DG, Gonçalves Basso F, Hebling J, De Souza Costa CA. Effect of hydrogen-peroxide-mediated oxidative stress on human dental pulp cells. J Dent. 2015;43(6):750–6.

Reconocimiento-NoComercial-CompartirIgual
CC BY-NC-SA
Esta licencia permite a otros entremezclar, ajustar y construir a partir de su obra con fines no comerciales, siempre y cuando le reconozcan la autorÍa y sus nuevas creaciones estÉn bajo una licencia con los mismos tÉrminos.











