Parameters for the germination of elite fungal biocontrol strains
Main Article Content
Abstract
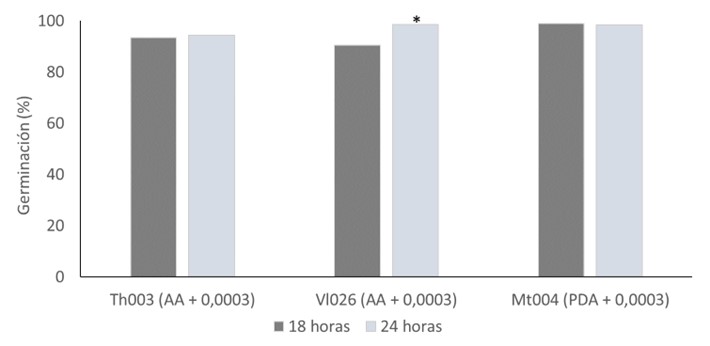
The marketing of microbial pesticides has increased exponentially in recent years. Almost 70% of the products marketed use as active ingredient conidia of biocontrol fungi, and One of the most frequently used criteria to evaluate the quality of these products is conidial germination test, Specifically, this methodology is used to select and assess the viability and vigor of elite strains with biological potential. Some of the factors that influence conidial germination over time, include the selection of culture medium, temperature, incubation time, and the addition of germination synchronizing agents. The optimization for each elite strain allows the characterization of a microorganism behavior over time, ensuring its biological activity and effective management as a biological resource for commercial purposes. The main objective of this study was to identify the optimal conditions (culture media, incubation time, and synchronizing agent concentration) for the germination of three elite strains: Trichoderma koningiopsis (Th003), Lecanicillium lecanii (Vl026), and Metarhizium robertsii (Mt004), which are active ingredients in biopesticides. As a result, the optimal conditions selected are as follows: agar-water medium with 0.0003 % benomyl for Th003 with an incubation period of 18 hours, agar-water medium with 0.0003 % benomyl for Vl026 with an incubation period of 24 hours, and PDA agar supplemented with 0.0003 % benomyl for Mt004 with an incubation period of 18 hours. These conditions will allow a precise evaluation of the germination of the biological control agents ensuring over time their viability and vigor as active ingredients in biopesticides.
Downloads
Metrics
Article Details

This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License.
The authors who publish in Siembra know and accept the following conditions:
- Authors retain the copyright and grant Siembra the right of first publication of the work, under the Creative Commons Attribution License. Third parties are allowed to use what has been published as long as they refer to the author or authors of the work and its publication in this journal.
![]() This content is licensed under a Creative Commons Attribution-Noncommercial 4.0 International (CC BY-NC 4.0).
This content is licensed under a Creative Commons Attribution-Noncommercial 4.0 International (CC BY-NC 4.0).
- Authors maintain the copyright and guarantee Siembra the right to publish the manuscript through the channels it considers appropriate.
- Authors may establish on their own additional agreements for the non-exclusive distribution of the version of the work published in Siembra, acknowledging their initial publication in the same, such as in institutional repositories.
- Authors are authorized to disseminate their work electronically once the manuscript is accepted for publication.
References
Afifah, L., Desriana, R., Kurniati, A., y Maryana, R. (2020). Viability of entomopathogenic fungi Metarhizium anisopliae (Metsch) Sorokin in some alternative media and different shelf-life. International Journal of Agriculture System, 8(2),108-118. http://dx.doi.org/10.20956/ijas.v8i2.2478
Alves, S. B., Pereira, R. M., Stimac, J. L., y Vieira, S. A. (1996). Delayed germination of Beauveria bassiana conidia after prolonged storage at low, above-freezing temperatures. Biocontrol Science and Technology, 6(4), 575-582. https://doi.org/10.1080/09583159631217 DOI: https://doi.org/10.1080/09583159631217
Brunner-Mendoza, C., Reyes-Montes, M. del R., Moonjely, S., Bidochka, M. J., y Toriello, C. (2019). A review on the genus Metarhizium as an entomopathogenic microbial biocontrol agent with emphasis on its use and utility in Mexico. Biocontrol Science and Technology, 29(1), 83-102. https://doi.org/10.1080/09583157.2018.1531111 DOI: https://doi.org/10.1080/09583157.2018.1531111
Castellanos González, L., Monroy Gonzalez, H. H., y Rivera Ochoa, X. G. (2022). Wilting by Fusarium oxysporum Schlthl in masaguaro (Pseudosamanea guachapele) (Kunth). INGE CUC, 19(1), 11-21. https://doi.org/10.17981/ingecuc.19.1.2023.02 DOI: https://doi.org/10.17981/ingecuc.19.1.2023.02
Cotes Prado, A. M. (ed.). (2018). Control biológico de fitopatógenos, insectos y ácaros: aplicaciones y perspectivas. Vol. 2. Agrosavia. https://doi.org/10.21930/agrosavia.investigation.7402544 DOI: https://doi.org/10.21930/agrosavia.investigation.7402544
Daryaei, A., Jones, E. E., Glare, T. R., y Falloon, R. E. (2016). Biological fitness of Trichoderma atroviride during long-term storage, after production in different culture conditions. Biocontrol Science and Technology, 26(1), 86-103. https://doi.org/10.1080/09583157.2015.1077929 DOI: https://doi.org/10.1080/09583157.2015.1077929
Diego-Nava, F., Granados-Echegoyen, C., Ruíz-Vega, J., Aquino-Bolaños, T., Pérez-Pacheco, R., Díaz-Ramos, A., Alonso-Hernández, N., Arroyo-Balán, F., y López-Hernández, M. B. (2023). Functional and quality assessment of a spore harvester for entomopathogenic fungi for biopesticide production. AgriEngineering, 5(2), 801-813. https://doi.org/10.3390/agriengineering5020049 DOI: https://doi.org/10.3390/agriengineering5020049
Faria, M. R. de, y Wraight, S. P. (2007). Mycoinsecticides and Mycoacaricides: A comprehensive list with worldwide coverage and international classification of formulation types. Biological Control, 43(3), 237-256. https://doi.org/10.1016/j.biocontrol.2007.08.001 DOI: https://doi.org/10.1016/j.biocontrol.2007.08.001
Faria, M., Hotchkiss, J. H., Hajek, A. E., y Wraight, S. P. (2010). Debilitation in conidia of the entomopathogenic fungi Beauveria bassiana and Metarhizium anisopliae and implication with respect to viability determinations and mycopesticide quality assessments. Journal of Invertebrate Pathology, 105(1), 74-83. https://doi.org/10.1016/j.jip.2010.05.011 DOI: https://doi.org/10.1016/j.jip.2010.05.011
Francisco, E. A., Mochi, D. A., Correia, A. do C. B., y Monteiro, A. C. (2006). Influence of culture media in viability test of conidia of entomopathogenic fungi. Ciência Rural, 36(4), 1309-1312. https://doi.org/10.1590/S0103-84782006000400043 DOI: https://doi.org/10.1590/S0103-84782006000400043
Gilchrist, M. A., Sulsky, D. L., y Pringle, A. (2006). Identifying fitness and optimal life‐history strategies for an asexual filamentous fungus. Evolution, 60(5), 970-979. https://doi.org/10.1111/j.0014-3820.2006.tb01175.x DOI: https://doi.org/10.1111/j.0014-3820.2006.tb01175.x
Hsia, I. C. C., Islam, M. T., Ibrahim, Y., Howand, T.Y., Omar, D. (2014). Evaluation of conidial viability of entomopathogenic fungi as influenced by temperature and additive. International Journal of Agriculture & Biology, 16(1), 146-152. https://www.fspublishers.org/Issue.php?categoryID=122
Inglis, G. D., Enkerli, J., y Goettel, M. S. (2012). Laboratory techniques used for entomopathogenic fungi: Hypocreales. En L. A. Lacey (ed.), Manual of Techniques in Invertebrate Pathology (2nd ed.) (pp. 189-253). Elsevier. https://doi.org/10.1016/B978-0-12-386899-2.00007-5 DOI: https://doi.org/10.1016/B978-0-12-386899-2.00007-5
Jackson, M. A. (1997). Optimizing nutritional conditions for the liquid culture production of effective fungal biological control agents. Journal of Industrial Microbiology and Biotechnology, 19, 180-187. https://doi.org/10.1038/sj.jim.2900426 DOI: https://doi.org/10.1038/sj.jim.2900426
Kala, S., Sogan, N., Agarwal, A., Naik, S. N., Patanjali, P. K., y Kumar, J. (2020). Biopesticides: formulations and delivery techniques. En C. Egbuna, y B. Sawicka (eds.), Natural remedies for pest, disease and weed control (pp. 209-220). Elsevier. https://doi.org/10.1016/B978-0-12-819304-4.00018-X DOI: https://doi.org/10.1016/B978-0-12-819304-4.00018-X
Le Grand, M., y Cliquet, S. (2013). Impact of culture age on conidial germination, desiccation and UV tolerance of entomopathogenic fungi. Biocontrol Science and Technology, 23(7), 847-859. https://doi.org/10.1080/09583157.2013.802289 DOI: https://doi.org/10.1080/09583157.2013.802289
Lopes, R. B., y Faria, M. (2019). Influence of two formulation types and moisture levels on the storage stability and insecticidal activity of Beauveria bassiana. Biocontrol Science and Technology, 29(5), 437-450. https://doi.org/10.1080/09583157.2019.1566436 DOI: https://doi.org/10.1080/09583157.2019.1566436
McGuire, A. v., y Northfield, T. D. (2020). Tropical occurrence and agricultural importance of Beauveria bassiana and Metarhizium anisopliae. Frontiers in Sustainable Food Systems, 4, 6. https://doi.org/10.3389/fsufs.2020.00006 DOI: https://doi.org/10.3389/fsufs.2020.00006
Meirelles, L. N., Mesquita, E., Corrêa, T. A., Bitencourt, R. de O. B., Oliveira, J. L., Fraceto, L. F., Camargo, M. G., y Bittencourt, V. R. E. P. (2023). Encapsulation of entomopathogenic fungal conidia: evaluation of stability and control potential of Rhipicephalus microplus. Ticks and Tick-Borne Diseases, 14(4), 102184. https://doi.org/10.1016/j.ttbdis.2023.102184 DOI: https://doi.org/10.1016/j.ttbdis.2023.102184
Miranda-Hernández, F., Angel-Cuapio, A., y Loera-Corral, O. (2017). Production of Fungal Spores for Biological Control. En A. Pandey, S. Negi, y C. R. Soccol (eds.), Current Developments in Biotechnology and Bioengineering (pp. 757–779). Elsevier. https://doi.org/10.1016/B978-0-444-63662-1.00033-6 DOI: https://doi.org/10.1016/B978-0-444-63662-1.00033-6
Mukhopadhyay, R., y Kumar, D. (2020). Trichoderma: a beneficial antifungal agent and insights into its mechanism of biocontrol potential. Egyptian Journal of Biological Pest Control, 30, 133. https://doi.org/10.1186/s41938-020-00333-x DOI: https://doi.org/10.1186/s41938-020-00333-x
Oliveira, D. G. P., Pauli, G., Mascarin, G. M., y Delalibera, I. (2015). A protocol for determination of conidial viability of the fungal entomopathogens Beauveria bassiana and Metarhizium anisopliae from commercial products. Journal of Microbiological Methods, 119, 44-52. https://doi.org/10.1016/j.mimet.2015.09.021 DOI: https://doi.org/10.1016/j.mimet.2015.09.021
Pathan, E. K., Patil, A. V., & Deshpande, M. V. (2019). Bioprospecting of fungal entomo-and myco-pathogens. En T. Satyanarayana, S. K. Deshmukh, y M. V. Deshpande (eds.), Advancing Frontiers in Mycology & Mycotechnology: Basic and Applied Aspects of Fungi (pp. 497-513). Springer Nature eBookhttps://doi.org/10.1007/978-981-13-9349-5_20 DOI: https://doi.org/10.1007/978-981-13-9349-5_20
Permadi, M. A., Mukhlis, Samosir, B. S., Siregar, D. Y., y Wayni, M. (2020). Physiology characterization of entomopathogenic fungi Beauveria bassiana and Metarhizium anisopliae on different carbohydrate sources. Journal of Physics: Conference Series, 1477(7), 072007. https://doi.org/10.1088/1742-6596/1477/7/072007 DOI: https://doi.org/10.1088/1742-6596/1477/7/072007
Rangel, D. E. N., Braga, G. U. L., Anderson, A. J., y Roberts, D. W. (2005). Variability in conidial thermotolerance of Metarhizium anisopliae isolates from different geographic origins. Journal of Invertebrate Pathology, 88(2), 116-125. https://doi.org/10.1016/j.jip.2004.11.007 DOI: https://doi.org/10.1016/j.jip.2004.11.007
Rodríguez-León, J. A., Domenech, F., León, M., Méndez, T., Rodríguez, D. E., y Pandey, A. (1999). Production of spores of Trichoderma harzianum on sugar cane molasses and bagasse pith in solid state fermentation for biocontrol. Brazilian Archives of Biology and Technology, 42(1). https://doi.org/10.1590/S1516-89131999000100010 DOI: https://doi.org/10.1590/S1516-89131999000100010
Saldaña-Mendoza, S. A., Pacios-Michelena, S., Palacios-Ponce, A. S., Chávez-González, M. L., y Aguilar, C. N. (2023). Trichoderma as a biological control agent: mechanisms of action, benefits for crops and development of formulations. World Journal of Microbiology and Biotechnology, 39, 269.https://doi.org/10.1007/s11274-023-03695-0 DOI: https://doi.org/10.1007/s11274-023-03695-0
Santos Díaz, A. M., Grijalba Bernal, E. P., Torres Torres, L., y Uribe Gutiérrez, L. A. (2022). Plaguicidas microbianos: control y aseguramiento de calidad. Agrosavia. https://doi.org/10.21930/agrosavia.manual.7405125 DOI: https://doi.org/10.21930/agrosavia.manual.7405125
Stentelaire, C., Antoine, N., Cabrol, C., Feron, G., y Durand, A. (2001). Development of a rapid and highly sensitive biochemical method for the measurement of fungal spore viability. An alternative to the CFU method. Enzyme and Microbial Technology, 29, 560-566. https://doi.org/10.1016/S0141-0229(01)00432-X DOI: https://doi.org/10.1016/S0141-0229(01)00432-X
Yáñez, M., y France, A. 2010. Effects of fungicides on the development of the entomopathogenic fungus Metarhizium anisopliae var. anisopliae. Chilean Journal of Agricultural Research, 70(3), 390-398. http://dx.doi.org/10.4067/S0718-58392010000300006 DOI: https://doi.org/10.4067/S0718-58392010000300006
Zaki, O., Weekers, F., Thonart, P., Tesch, E., Kuenemann, P., y Jacques, P. (2020). Limiting factors of mycopesticide development. Biological Control, 144, 104220. https://doi.org/10.1016/j.biocontrol.2020.104220 DOI: https://doi.org/10.1016/j.biocontrol.2020.104220